Background: Asparaginase (ASP) is critical in acute lymphoblastic leukemia (ALL) and lymphoma (LLy) therapy. Hyperglycemia following ASP is described, but hypoglycemia is rarely reported. We previously reported on the risk factors, prevalence, duration, severity, recurrence, and outcomes of children at Texas Children's Hospital (TCH) developing ASP-induced hypoglycemia (defined as blood glucose [BG] <70mg/dL). Out of 723 ALL or LLy patients, there was a 7.6% prevalence of ASP-induced hypoglycemia in pediatric patients with ALL (n=50) or LLy (n=5), and occurred with both pegylated and Erwinia formulations of ASP. Given the median hypoglycemia duration was 11 days in our cohort and 76% of our cohort experience recurrent hypoglycemia with subsequent ASP doses, we here report the management details of these patients to provide guidance for other providers.
Design/Method: Descriptive analysis of cohort of children with ASP-induced hypoglycemia at TCH between 6/1/2017 and 6/30/2022. Following identification of diagnosis and frequency of hypoglycemia events, we reviewed EMR to collect information on treatments utilized including evaluation with a registered dietician (RD), dietary changes recommended during hypoglycemia, and utilization of cornstarch supplementation, continuous feedings or use of continuous glucose monitor (CGM). Hypoglycemia severity was determined based on lowest recorded BG (Stages 1-2) and need for hospitalization or seizures (Stage 3).
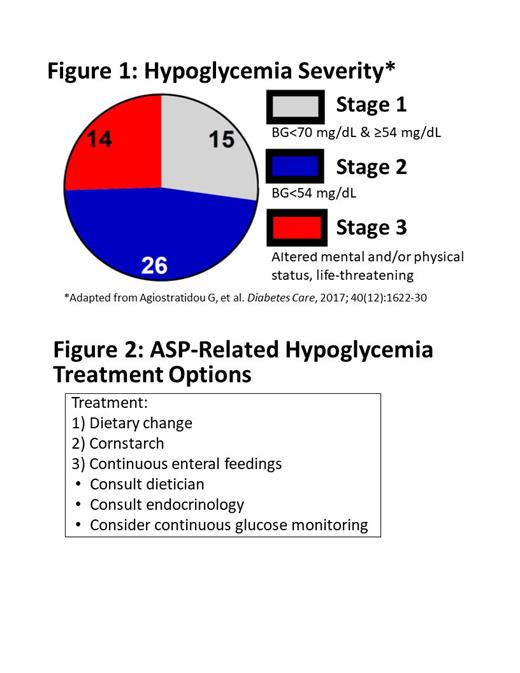
Results: 26% of patients had Stage 1 hypoglycemia (n=14), 47%% had Stage 2 hypoglycemia (n=26), and 25% had Stage 3 hypoglycemia (n=15) (Figure 1). Treatment to prevent symptomatic/severe hypoglycemia included: 1) dietary change (53%, n=29), 2) cornstarch (29%, n=16), and 3) continuous enteral feedings (13%, n=7). Dietary change included education on complex carbohydrates and a consistent carbohydrate diet. Cornstarch dosing was determined in consultation with endocrinology, and typically doses were provided prior to periods of prolonged fasting (e.g., overnight, NPO). Enteral feedings were administered through daytime bolus feedings and continuous overnight feedings, with the goal of a consistent carbohydrate infusion and maintaining BG levels >70 mg/dL. Five patients (9%) required all 3 interventions. Dietary education and subsequent follow up was completed by an RD in 54% of patients (n=30), and among patients with multiple recurrences, 67% had RD involvement (n=37). Nine patients in our cohort (16%) used CGM to closely monitor adequacy of carbohydrate intake. The primary team evaluated home BG logs weekly. Despite frequent treatment or nutritional management, only 39 (69%) of individuals had formal endocrine consultation, most commonly those with Stage 3 hypoglycemia (13 of 14; 93%). Patients with Stage 3 hypoglycemia tended to be younger (median age 2.8 years), tended to require the most intensive therapy (47% Vs 38% Vs 15% requiring medical therapy for Stages 3 ,2, and 1), and were more often to require nutritional counseling (79% Vs 46% Vs 42%). Only one patient had ASP held due primarily to hypoglycemia (Stage 3 hypoglycemia), while another patient had it held primarily for ASP-induced pancreatitis but had Stage 1 hypoglycemia. The overall survival of our cohort was 80% (85% if excluding patients receiving ASP for relapsed disease), with 3.5 years' median follow up.
Conclusion: ASP-induced hypoglycemia is a fairly common, recurrent, and possibly severe potential side effect in children receiving treatment for ALL or LLy. Reassuringly, patients completed treatment without worsened survival. However, some patients required intensive therapy throughout the duration of ASP treatments and required multi-modal therapy and intensive monitoring (Figure 2). Our systematic approach was effective at reducing severe events and hospitalizations, without compromising survival after therapy completion. We recommend other centers consider close monitoring and developing treatment algorithms to detect and manage ASP-induced hypoglycemia.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal